Game Play
Objective of the game
Imagine you are a chemist at a large chemical company. Your department is requesting that you develop environmentally friendly chemicals. As a part of this initiative, you were asked to design a detergent that not only has an optimal cleaning performance, but also is safer for humans and environment. If your product is successful, it will be widely produced and distributed in major chain stores.
Goal
Your task is to design a benign detergent that has optimal cleaning capabilities. As a chemist, you can thoughtfully design a chemical to have certain characteristics (e.g., be an efficient cleaning agent or have a reduced toxicity). You can achieve this by manipulating its physical and chemical properties. Your goal is to adjust these properties such that the detergent does not cause harmful (adverse) effects to humans and fish without sacrificing the product’s cleaning ability.
Game Levels
This game has two levels:
- In Level 1, you will address detergent toxicity by limiting its absorption through the membranes. You will design a detergent that will not be absorbed into the body through skin, lungs, or intestines, and will not harm fish in bodies of water, like lakes or ponds.
- In Level 2 the detergent is absorbed into the body. This is problematic, but as a chemist, you are able to minimize its toxicity and effects on the body. Note: Distribution, Metabolism, and Excretion are processes that occur when a chemical enters the body. Taken together with Absorption, the processes are referred to as “ADME” (Absorption-Distribution-Metabolism-Excretion).
Information on detergents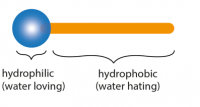
Detergents are substances that are used to remove grease and grime. Their performance is due to their unique structure. One end of their molecule, called head, is attracted to water (hydrophilic), while the other end, called tail, is attracted to dirt and grease (lipophilic).
Detergent heads and tails can vary, and they are made of different chemical functional groups.

For example, tails consist of a hydrocarbon chain, which can be branched, linear, or aromatic. Heads, on the other hand, may include sulfonate, phosphate, sulfate or carboxylate functionalgroup which define surfactant’s character (antimicrobial & antifungal hand soap, versus cleaner or fragrance carrier).
The most common use is grease removal. Once the grease is detected, it is surrounded by hydrophobic (lipophilic) tails forming a structure called micelle. Micelle are typically spherical in shape and allow greasy compounds, which are normally insoluble, to dissolve.
Real world Application
- The computer game simulates the real-world constraints that may affect chemical product development as the student designs a novel product. It is important to think about the product holistically, and find a ‘sweet spot’ for reduced toxicity and increased performance.
- The game is made based on real world data.
Pre-Game Question
Speculate how molecular weight, lipid solubility, physical state (solid vs liquid) and vapor pressure of the detergent will impact absorption into the body.