Aqueous and Lipid Solubility
Learning Outcomes: By the end of this module, the student will be able to:
- Define Kow and logP.
- Associate related chemical properties with solubility.
- Use ExpoBox and external websites and data to learn more about physicochemical properties that determine solubility and chemical transport.
Background and Information: Solubility of a chemical is determined by its structure and solution conditions. Aqueous solubility is important because, except for gases, chemical agents in the environment are transported and made available to living systems as aqueous solutions. Chemicals are either hydrophilic (water-soluble) or hydrophobic (water-insoluble). Aqueous solubility is measured by Kow (also denoted by “P” in some references). Kow is a shorthand term for the octanol-water partition coefficient. It measures relative solubility of a chemical with water (a polar molecule) and compared with octanol (a non-polar molecule similar to biological phospholipids found in cell walls/membranes). Kow provides information on how the chemical is likely to partition in biological organisms. For this module, we are focusing on the toxicology applications of Kow for purposes of chemical design; other resources exist for understanding Kow as it relates to other uses such as environmental fate, chemical behavior and hazard. A lower Kow indicates a preference for water over oily solvents; a higher Kow means it is more lipid-loving than water loving (“lipophilic”). Kow is useful because it predicts the preference a chemical has for lipids and for other organic media such as soil organic matter. In regard to lipid solubility, it is an important measure of the relative safety of chemicals because cells have lipid membrane/cell wall barriers, and so movement of chemicals across membranes and into organs and cells is dependent on its lipophilicity (lipid-solubility). The lipophilicity of a chemical is measured by log P (or log Kow- both expressed as a base -10 exponent. A P value of 1000 or 103 has a log P value of 3). In general, liquids with a log P between 2-4 tend to absorb well through skin. Likewise, a value that exceeds 5 to 6 does not absorb well, and tends to bioconcentrate in the lipid portion of the membrane.
Chemical designers rely on many factors when determining appropriate chemical design choices that result in low toxicity products including polarity, molecular size, surface area, and other descriptors of solute-solvent interactions. Aqueous and lipid solubility are just a few of several physiochemical properties that can help us understand how likely a chemical will be absorbed through biological membranes. Solubility data is available for many commonly used chemicals through reliable sources in the literature, such as Merck Index and Beilstein. The Interstate Chemicals Clearinghouse (IC2) framework, guiding development and use of safer chemicals and products, describes additional chemical properties that must be considered for reaching Green Chemistry Principle #4: Chemical products should be designed to preserve efficacy of function while reducing toxicity. In addition, several screening tools, such as EPA’s EPI SuiteTM are available to assist in estimating physical/chemical and environmental fate properties when data is not available or when one is designing a new chemical.
Video: Dr. Paul Anastas, Director, Center for Green Chemistry and Green Engineering & Teresa and H. John Heinz III Professor in the Practice of Chemistry for the Environment, School of Forestry & Environmental Studies, Yale University
Assignment:
Visit the following webpage: http://www2.epa.gov/expobox
Under the “Media” Column, click “Aquatic Biota” and then select the “Fate & Transport” Tab.
Click on the link for “Physicochemical/Environmental Factors” and look over the physicochemical properties in addition to the Kow or logP that can determine the solubility and transport of chemicals.
Many of these properties that help predict and determine the fate and transport a chemical has in the environment are also used in determining the biological activity (ADME - Adsorption, Distribution, Metabolism, Excretio
n) of chemicals in living organisms including us. In addition to Kow are factors such as the half-life which can be an index of persistence, Henry’s law constant (KH) which is an index of air/water partitioning. Consideration of all physicochemical properties is important, however the Kow of a chemical is very useful for environmental assessments. If the Kow of a compound is known, it can serve as a reliable predictor to estimate the Kow value for other chemicals within that class of compounds.
Consider the following data provided by the EPA investigating “Basic Concepts of Contaminant Sorption at Hazardous Waste
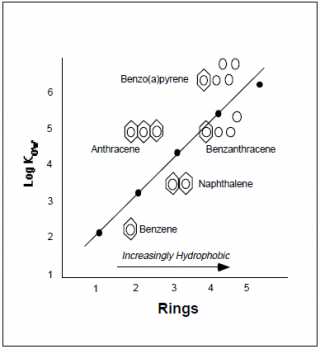
Sites” (see References):
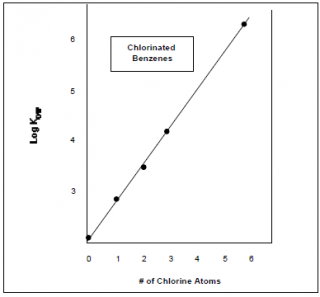
Figure A. Relationship of Molecular Structure to Hydrophobicity Figure B. Relationship of Molecular Structure to Kow
In these two examples, the Kow can be reliably predicted from the molecular structures. In the first graph it is correlated with the number of chlorine atoms, while in the second it is correlated with the number aromatic rings associated with the chemical. Now let us consider these concepts looking at some examples of a chemical class called Polychlorinated biphenyl’s (PCB’s) that were manufactured and used mainly as flame-retardant lubricants in the U.S. until they were banned by Congress in 1979.
Visit https://pubchem.ncbi.nlm.nih.gov/search/ and perform a search on each of the six chemicals to determine their respective Kow. How would you rank these chemicals from lowest to highest Kow?
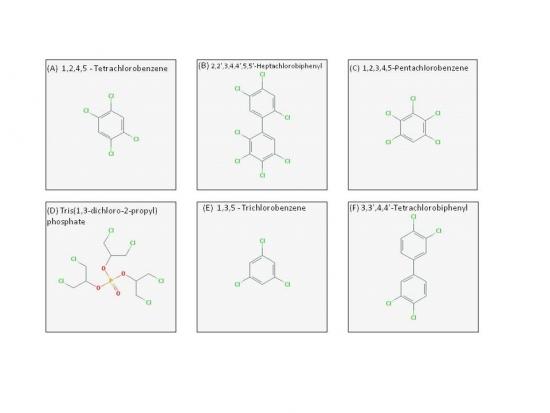
Assignment answer found HERE: Aqueous and Lipid Solubility Answer Sheet
Additional Assignments:
- Read this National Toxicology Program report for chlorinated paraffins found here:http://ntp.niehs.nih.gov/ntp/roc/content/profiles/chlorinatedparaffins.pdf - summarize the role of lipid or aqueous solubility in the designation of its carcinogenicity in animal studies. How do these properties impact how the molecule can enter into humans and how it may impact the health of living animals?
- Describe what a safe chemical would “look” like or what properties it would have using the following physicochemical properties: logP, molecular weight, molecular size, surface area, and membrane permeability.
Click here to evaluate this module.
Resources:
- American Chemical Society. (2015) Green Chemistry Principle #4. Retrieved fromhttp://www.acs.org/content/acs/en/greenchemistry/what-is-green-chemistry/principles/gc-principle-of-the-month-4.html#articleContent_headingtext_2
- Mergel, M. (2001). Chlorinated Parrafins. Retrieved fromhttp://www.toxipedia.org/display/toxipedia/Chlorinated+Paraffins
- Piwoni, M. D., & Keeley, J. W. (1990). Basic concepts of contaminant sorption at hazardous waste sites. Retrieved from http://www2.epa.gov/sites/production/files/2015-06/documents/basic_concepts_sorption_haz_site.pdf
- Price, D. A., Blagg, J., Greene, N., & Wager, T. (2009). Physicochemical drug properties associated with in vivo toxicological outcomes: a review. Expert Opinion on Drug Metabolism & Toxicology 5(8), 921-931.
- PubChem. (2016). Retrieved from https://pubchem.ncbi.nlm.nih.gov/
This material is based upon work supported by the NSF Division of Chemistry and the Environmental Protection Agency under Grant No. 1339637.